Meet with our product experts in one-on-one virtual sessions
Set Up a MeetingActualizaciones
Product Name - Siponimod Hemifumarate
US DMF filed
Product Name - Bempedoic Acid
US DMF filed
Product Name - Risperidone [Form-1]
Brazil DMF Filed
Product Name - Sacubitril + Valsartan Crystalline
Brazil DMF Filed
Product Name - Ramipril
Brazil DMF Filed
Product Name - Levofloxacin Hemihydrate
Brazil DMF Filed
Product Name - Voriconozole
Brazil DMF Filed
Product Name - Apremilast [Amorphous]
Brazil DMF Filed
Product Name - Apremilast [Form-B]
Brazil DMF Filed
Product Name - Siponimod Hemifumarate
Brazil DMF Filed
Product Name - Metoprolol Succinate
China DMF filed
Product Name - Dapagliflozin PG Solvate
China DMF filed
Product Name - Pemetrexed Disodium Heptahydrate
China DMF filed
Product Name - Clopidogrel Bisulphate [Form-1]
Japan DMF filed
Product Name - Rivaroxaban
Japan DMF filed
Product Name - Lenalidomide [Form-A]
Japan DMF filed
Product Name - Naratriptan Hydrochloride
Japan DMF filed
Product Name - Fingolimod Hydrochloride
CEP DMF Filed
Product Name - Sugammadex Sodium
Canada DMF Filed
Product Name - Apremilast Form B
Korea DMF Filed
Product Name - Rabeprazole Sodium [Amorphous]
Korea DMF Filed
Product Name - Sumatriptan Succinate
Korea DMF Filed
Product Name - Enzalutamide
Korea DMF Filed
Product Name - Sacubitril/Valsartan
Korea DMF Filed
Product Name - Pioglitazone Hydrochloride
Korea DMF Filed
Product Name - Montelukast Sodium
Korea DMF Filed
Product Name - Eribulin Mesylate
Australia DMF Filed
Product Name - Molnupiravir
WHO DMF Filed
Product Name - Omeprazole Magnesium
CEP Granted
Product Name - Dasatinib Monohydrate
Japan DMF Filed
Product Name - Fexofenadine Hydrochloride (Form-I)
Brazil DMF Filed
Product Name - Dapagliflozin Amorphous
Brazil DMF Filed
Product Name - Losartan Potassium [Form-I]
Korea DMF Filed
Product Name - Losartan Potassium [Form-I]
Brazil DMF Filed
Product Name - Losartan Potassium [Form 1]
CEP Granted
Product Name - Capecitabine
Japan DMF filed
Product Name - Losartan Potassium
Japan DMF
Product Name - Ticagrelor
Russia DMF
Product Name - Escitalopram Oxalate
CEP Granted
Product Name - Sugammadex Sodium
Taiwan DMF
Product Name - Bempedoic Acid
Brazil DMF
Product Name - Lurasidone Hydrochloride
EU DMF
Product Name - Eribulin Mesylate
EU DMF
Product Name - Nilotinib Hydrochloride
USDMF
Product Name - Tofacitinib Citrate
USDMF
Product Name - Eribulin Mesylate
Canada DMF
Product Name - Palbociclib [Form-A]
Brazil DMF
Product Name - Metoprolol Succinate
CEP Granted
Product Name - Clopidogrel Bisulfate [Form-I] CIP
Brazil DMF
Product Name - Moxifloxacin Hydrochloride USP
Brazil DMF
Product Name - Fexofenadine Hydrochloride
Korea DMF
Product Name - Sitagliptin Phosphate Monohydrate
Korea DMF
Product Name - Clopidogrel Bisulfate [Form-II]
CEP Granted
Product Name - Sitagliptin Phosphate Monohydrate
CEP Granted
Product Name - Sumatriptan Succinate USP
USDMF
Product Name - Empagliflozin
Brazil DMF
Product Name - Atorvastatin Calcium Trihydrate (Form-1)
Brazil DMF
Product Name - Dimethyl Fumarate
Brazil DMF
Product Name - Atorvastatin Calcium Trihydrate (Form-1)
Japan DMF
Product Name - Clopidogrel Bisulfate [Form-II]
EUDMF
Product Name - Linagliptin
EUDMF
Product Name - Linagliptin
Brazil DMF
Product Name - Dimethyl Fumarate
EUDMF
Product Name - Nilotinib Hydrochloride Monohydrate
Brazil DMF
Product Name - Levocetirizine Dihydrochloride
Brazil DMF
Product Name - Clopidogrel Bisulphate (Form - II)
China DMF
Product Name - Ibrutinib [Form - A]
USDMF
Product Name - Ibrutinib [Form - A]
Brazil DMF
Product Name - Ketorolac Tromethamine
China DMF
Product Name - Capecitabine
Japan DMF
Product Name - Levetiracetam
Korea DMF filed
Product Name - Bendamustine HCl
Canada DMF filed
Product Name - Fexofenadine Form-1
CEP Granted
Product Name - Clopidogrel Bisulphate Form-2
CEP Granted
Product Name - Mirabegron
Europe ASMF filed
Product Name - Permethrin-25:75
CEP Granted
Product Name - Pregabalin
Korea DMF filed
Product Name - Ondansetron Hydrochloride
Korea DMF filed
Product Name - Sitagliptin Phosphate USP (monohydrate)
US DMF filed
Product Name - Hydroxychloroquine Sulfate
US DMF filed
Product Name - Favipiravir
US DMF filed
Product Name - Finasteride (Form-I)
Brazil DMF Available
Product Name - Losartan Potassium
Japan DMF filed
Product Name - Clopidogrel Bi-sulfate (Form-II)
Japan DMF filed
Product Name - Amlodipine Besilate
China DMF filed
Product Name - Abiraterone Acetate
Japan DMF filed
Product Name - Naproxen Sodium
Brazil DMF Available
Product Name - Naproxen
Brazil DMF Available
Product Name - Sitagliptin Phosphate Ph.Eur (monohydrate)
CEP Granted
Product Name - Apalutamide (Amorphous)
US DMF filed
Product Name - Finasteride (Form-I)
China DMF filed
Product Name - Ondansetron base
Brazil DMF Available
Product Name - Rabeprazole Sodium [Amorphous]
Japan DMF filed
Product Name - Dabigatran Etexilate Mesylate
Korea DMF filed
Product Name - Bendamustine Hydrochloride
Brazil DMF Available
Product Name - Sitagliptin Phosphate Monohydrate USP
Brazil DMF Available
Product Name - Rabeprazole Sodium [Amorphous]
China DMF filed
Product Name - Pregabalin
China DMF filed
Product Name - Fingolimod Hydrochloride
China DMF filed
Product Name - Dapagliflozin (Amorphous)
China DMF filed
Product Name - Olaparib
US DMF filed
Product Name - Diroximel Fumarate
US DMF filed
Product Name - Sitagliptin Phosphate Monohydrate USP
US DMF filed
Product Name - Lurasidone HCl
Brazil DMF Available
Product Name - Atorvastatin Calcium Trihydrate [Form-I] ATN process
China DMF filed
Product Name - Rivaroxaban
Korea DMF filed
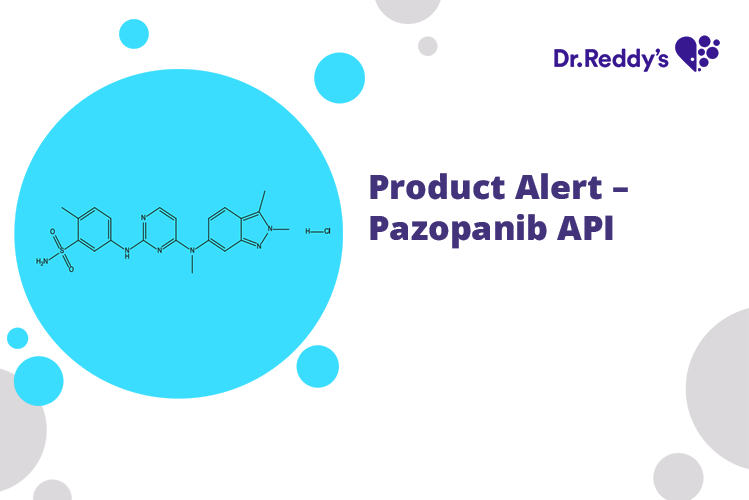
Product Name - Pazopanib Hydrochloride
US DMF filed
Product Name - Pregabalin
US DMF filed
Product Name - Dimethyl Fumarate
EDMF filed
Product Name - Olanzapine
Korea DMF filed
Product Name - Ropinirole
CEP Granted
Product Name - Rivastigmine HT P-1
CEP Granted
Product Name - Esomeprazole Magnesium Trihydrate
CEP Granted
Product Name - Eribulin Fragment A
US DMF filed
Product Name - Lenvatinib mesylate (Form C)
US DMF filed
Product Name - Dapagliflozin PG Solvate [Micronized]
Korea DMF filed
Product Name - Clopidogrel Bisulphate [Form-II]
Korea DMF filed
Product Name - Olanzapine [Form-I]
Korea DMF filed
Product Name - Levofloxacin (USP) Hemihydrate
Korea DMF filed
Product Name - Azacitidine
Brazil DMF Available
Product Name - Pregabalin
Brazil DMF Available
Product Name - Diluted Everolimus
Brazil DMF Available
Product Name - Atorvastatin Calcium Trihydrate [Form-I] USP
Brazil DMF Available
Product Name - Midostaurin - Crystalline Form II
Brazil DMF Available
Product Name - Edaravone
CEP Granted
Product Name - Ranolazine
CEP Granted
Product Name - Enzalutamide
Brazil DMF Available
Product Name - Metoprolol Succinate FW
Brazil DMF Available
Product Name - Semaglutide CJ
US DMF filed
Product Name - Ibandronate Sodium Monohydrate [Form-B]
Brazil DMF Available
Product Name - Enalaprilat
US DMF filed
Product Name - Atorvastatin Calcium [Form-P]
Brazil DMF Available
Product Name - Dabigatran Etexilate Mesilate [Form-2]
Brazil DMF Available
Product Name - Dutasteride [Form-II]
Brazil DMF Available
Product Name - Cinacalcet HCl
China DMF filed
Product Name - Mirabegron (Alpha)
China DMF filed
Product Name - Quetiapine Fumarate (Ph.Eur)
Brazil DMF Available
Product Name - Quetiapine Fumarate (USP)
Brazil DMF Available
Product Name - Rabeprazole Sodium (Form-Y)
Brazil DMF Available
Product Name - Apixaban
China DMF filed
Product Name - Pioglitazone Hydrochloride
Japan DMF filed
Product Name - Atorvastatin Calcium (Amorphous)
Brazil DMF Available
Product Name - Eribulin Mesylate
US DMF filed
Product Name - Sacubitril/Valsartan
China DMF filed
Product Name - Gemcitabine Hydrochloride
China DMF filed
Product Name - Azacitidine
China DMF filed
Product Name - Rivaroxaban (Ph.Eur)
Brazil DMF Available
Product Name - Lenalidomide (LEU1)
Brazil DMF Available
Product Name - Lenalidomide [Form-A]
CEP Granted
Product Name - Moxifloxacin hydrochloride
Brazil DMF Available
Product Name - Mirabegron [Alpha]
Brazil DMF Available
Product Name - Sitagliptin Phosphate MH
Brazil DMF Available
Product Name - Valsartan [Compacted]
Brazil DMF Available
Product Name - Dasatinib SPG Solvate
Brazil DMF Available
Product Name - Atorvastatin Calcium Trihydrate (Form-1)
Canada DMF filed
Product Name - Valsartan
Canada DMF filed
Product Name - Esclicabazepine Acetate
Europe ASMF
Product Name - Abiraterone acetate
Europe ASMF
Product Name - Ticagrelor
Brazil DMF Available
Product Name - Levetiracetam
Brazil DMF Available
Product Name - Clopidogrel Bisulphate Form-1
Brazil DMF Available
Product Name - Sugammadex Sodium
Brazil DMF Available
Product Name - Sitagliptin HCl (CIP)
Korea DMF filed
Product Name - Icatibant Acetate
Europe ASMF
Product Name - Azacitidine
Japan DMF filed
Product Name - Liraglutide
US DMF filed
Product Name - Montelukast Sodium Ph.Eur.
Brazil DMF Available
Product Name - Gemcitabine Hydrochloride
Brazil DMF Available
Product Name - Sertraline HCl (SET4L)
Brazil DMF Available
Product Name - Rivaraoxaban
CEP Granted
Product Name - Keterolac Tromethamine
Brazil DMF Available
Product Name - Ondansetron Hydrochloride
Brazil DMF Available
Product Name - Sitagliptin Phosphate Anhydrous
Brazil DMF Available
Product Name - Apixaban
Brazil DMF Available
Product Name - Naratriptan Hydrochloride
Brazil DMF Available
Product Name - Valsartan [micronized]
Brazil DMF Available
Product Name - Pregabalin
China DMF filed
Product Name - Tizanidine
CEP Granted
Product Name - Losartan K
Brazil DMF Available
Product Name - Ondansetron HCl
China DMF filed
Product Name - Canagliflozin Hemihydrate
China DMF filed
Product Name - Raloxifene HCl
Korea DMF filed
Product Name - Escitalopram Oxalate
Korea DMF filed
Product Name - Sitagliptin Phosphate
Canada DMF filed
Product Name - Ticagrelor
CEP Granted
Product Name - Fexofenadine HCl ( form X)
Japan DMF filed
Product Name - Midostaurin
US DMF filed
Product Name - Divalproex Sodium
Brazil DMF Available
Product Name - Rivaroxaban
Brazil DMF Available
Product Name - Mirabegron Amorphous
Brazil DMF Available
Product Name - Sitagliptin Phosphate (Anhydrous)
China DMF filed
Product Name - Dutasteride (Form-II)
Canada DMF filed
Product Name - Rabeprazole Sodium Hydrate (form-Y) RBL process
CEP Granted
Product Name - Dapagliflozin Propane Diol
Brazil DMF Available
Product Name - Abiraterone Acetate
Brazil DMF Available
Product Name - Sugammadex Sodium
China DMF filed
Product Name - Lurasidone HCl
China DMF filed
Product Name - Raloxifene HCl
China DMF filed
Product Name - Apremilast (Form-B)
China DMF filed
Product Name - Empagliflozin [Crystalline]
Korea DMF filed
Product Name - Atorvastatin Calcium [Form-1] [ATN]
Korea DMF filed
Product Name - Rabeprazole Sodium (Amorphous)
CEP Granted
Product Name - Pregabalin
Japan DMF filed
Product Name - Famotidine
Japan DMF filed
Product Name - Lenalidomide
US DMF filed
Product Name - Clopidogrel Bisulfate
US DMF filed
Product Name - Roxadustat
US DMF filed
Product Name - Elagolix
US DMF filed
Product Name - Clopidogrel Bisulphate [Form-I] (CIP)
Brazil DMF Available
Product Name - Atorvastatin Calcium Trihydrate [Form-1] [ATN]
Brazil DMF Available
Product Name - Montelukast Sodium USP
Brazil DMF Available
Product Name - Pregabalin
Brazil DMF Available
Product Name - Capecitabine
Brazil DMF Available
Let’s discuss API sourcing

Join us at Hall - 9.1 | Booth - B48